
Changes in the PfEMP protein on the
malarial cell membrane :
Introduction:
The
ability of Plasmodium falciparum to reinfect previously exposed individuals is
due to the existence of phenotypically variant strains of the parasite in
endemic areas. The epidemiology of malaria is heavily dependent upon this
variance. Clinical studies have shown thatimmunity to malaria requires repeated
infections and is slow to develop, and that children under ten years of age are
the most susceptible to grave illness and fatality. This pathology is explained
by the diversity of P. Falciparum strains and the variant allelic forms of
parasite proteins that they produce. Cerebral malaria, a fatal form of the
disease, is caused by the aggregation of infected red blood cells adhering to
and blocking capillaries in the brain. Adults who have been exposed to variant
strains of the P. Falciparum and are able produce a sufficient humoral response
to parasite proteins involved in parasite cytoadherence. Children, however, have
not been immunologically exposed to this diverse body of parasite proteins.
Their inability to mount a strong immune response to variant strains can, in
extreme situations, result in fatality. Antigenic diversity of parasite strains
is a product of genetic recombination during both the sexual, vector phase and
the asexual amplification phase during human red blood cell infection.
Individual Parasite Diversity
Antigenic
diversity at the level of the individual parasite occurs during the course of
infection of a given individual. Variation occurs in the surface antigens of
infected red blood cells during the erythocytic schizogany phase of the parasite
life cycle. The sequestration of the malaria parasites in human red blood cells
during this phase of the cycle poses an extremely complex method for evasion to
the host immunity. First, infected RBCs do not induce CTL response due to their
lackof MHC expression. Second, parasite derived particles exposed on the surface
of the cell are highly variable, leading to the inability of the immune response
to produce adequate memory to the antigens. These molecules are associated with
PfEMP-1 proteins and undergo high clonal variation (2% per generation, in
culture).
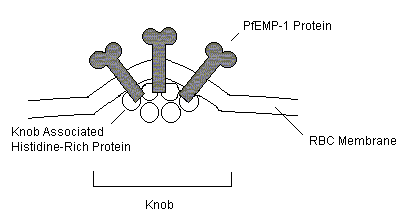
These proteins are produced by the parasite within the infected red blood cell,
and are transported to the cell surface. At the surface PfEMP-1 becomes
associated with Histidine-Rich Proteins (HRP) to form knob-like protrusions at
the membrane. PfEMP-1 is responsible for infected erythrocyte adherence. The
genes coding for PfEMP-1 have been characterized as the var genes, and are found
on a number of the parasiteís fourteen chromosomes. Individual parasite
genomes contain a diverse repertoire of 50-150 var genes. Because only a limited
number of the genes are expressed by each parasite, switches between different
loci of the gene during asexual reproduction can lead to the production of
extremely diverse PfEMP-1 proteins amongst the population. This variation is
immunologically important as it results in the hostís inability to produce
appropriate antibodies within the limited time frame of erythrocyte infection.
The specific mechanism of the gene expression regulation has not been
determined.
![]()