Inhaled Insulin: An
Overview
 About
the following article : This article is a
review paper published by the authors mentioned below. We at KEMates have had
nothing to do with editing the paper, except for formatting it (i.e. making the
headings more prominent etc..) and adding the photographs. The following article
has been placed in the public domain i.e. we can publish the article without
permission as long as we credit the original authors with it.
About
the following article : This article is a
review paper published by the authors mentioned below. We at KEMates have had
nothing to do with editing the paper, except for formatting it (i.e. making the
headings more prominent etc..) and adding the photographs. The following article
has been placed in the public domain i.e. we can publish the article without
permission as long as we credit the original authors with it.
John R. White, Jr., PA-C, PharmD, and R. Keith Campbell,
MBA, RPh, CDE
Introduction:
Various methods of insulin administration other than
injection have been sought since the discovery of insulin. For the past several
years, systems that deliver insulin via the pulmonary route have been developed
and evaluated. Based on available data, pulmonary insulin appears to be safe,
efficacious, and well accepted by patients. This article describes the
technology behind several of these pulmonary administration systems and outlines
the most recent data from clinical trials evaluating pulmonary insulin.
History:
The first patient to receive a dose of insulin was
14-year-old, 65-lb Leonard Thompson. He received an impure injection of 15 ml,
which was described as “a thick brown muck,” on January 11, 1922. His blood
glucose fell slightly, and because of the impurities in the extract, he
developed an abscess at the site of one of his injections.1
Since then, a plethora of purer forms of insulin and
insulins with various time action profiles have been produced, approved, and
administered.2
Protamine zinc insulin was introduced in the 1930s. NPH
was introduced in the 1940s. The lente series was introduced in the 1950s.2
Advances in chromotography in the 1960s and 1970s led to the production of
highly purified insulins. In the 1980s, recombinant DNA technology was used to
produce human insulin. Insulin was the first drug ever produced by recombinant
technology.
More recently, DNA technology has led to the ability to
synthesize insulin analogs. To date, more than 300 insulin analogs have been
produced.2 While the purity of insulin has increased and the needle size for
injections has decreased, thus reducing the discomfort associated with
subcutaneous insulin injections, no method of insulin delivery other than
injection is currently available.
The concept of nasally administered insulin first
appeared in 1935.3 Unfortunately, low bioavailability and great variability in
absorption found in research done thus far have demonstrated that nasally
administered insulin is not particularly practical.
Several years ago, interest in the possibility of
administering insulin via the pulmonary route surfaced. Since that time, several
methods have evolved that may eventually bring this idea to fruition. This
article briefly reviews the physiological and pharmacological basis for
pulmonary insulin and discusses several of the more salient systems currently
being evaluated in the United States.
Rapidly acting
pulmonary insulin:
Recent technological advances have made it feasible to
deliver insulin to the alveolar space. Here, it is rapidly absorbed into the
alveolar capillaries and disbursed throughout the systemic circulation. Alveolar
epithelium measures ~100 m2 (the size of a tennis court).4 This extremely
vascularized surface is very permeable, making inhaled insulin an attractive
alternative to injections. The absorptive ability of the alveolar surface stands
in contrast to the thick layered mucosoa of the upper airways and the bronchial
tree, which are relatively impermeable to peptide drugs.
Until quite recently, insulins administered via the
pulmonary route in human studies were soluble, rapid-acting formulations.
Technology is now available that may allow for the pulmonary administration of
longer-acting, as well as rapid-acting, insulin compounds.
Inhalational
Systems:
Two of the more well-known and highly publicized
inhalation systems are those from Inhaled Therapeutics of San Carlos, Calif.,
which is working in collaboration with Pfizer and Aventis, and from Aradigm
Corporation of Hayward, Calif. These two systems use different technology to
deliver insulin via the pulmonary route.
1) The Inhaled Therapeutics
system :
 This device uses a fine-powdered formulation. The particle size used in this system is less
than 5 µm in diameter.4,5 Particles of this size are able to reach the deep
lung with slow, deep inhalation. Larger particles are more likely to become
lodged in the upper airway, while smaller particles will be partially exhaled.5
This device uses a fine-powdered formulation. The particle size used in this system is less
than 5 µm in diameter.4,5 Particles of this size are able to reach the deep
lung with slow, deep inhalation. Larger particles are more likely to become
lodged in the upper airway, while smaller particles will be partially exhaled.5
Inhaled Therapeutics uses a technology it developed
called “PulmoSol powder technology” to create the right-sized particles to
reach the deep lung. These particles are highly soluble and quickly dissolve
upon reaching the alveoli. They then pass a single cellular layer into the
circulation.
Powdered aerosolized particles can contain up to 95% pure
drug, in contrast to aqueous aerosols, which typically contain only 1 or 2% drug
and about 98% water. These powder aerosols carry approximately five times more
drug in a single breath than does a metered-dose inhaler system and much more
drug than do liquid or nebulizer systems.5
The PulmoSol glass
stabilization system :
This process creates chemically stable insulin particles. Using a fast-drying
technique, the system places insulin into an amorphous, glassy state. This state
has many of the properties of a liquid but the viscosity of a solid.5
Insulin from this system will be available in “blister
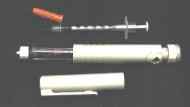
packs” and will remain stable at room temperature for up to 2 years. The
device used with the Inhaled Therapeutics system is the size of a mechanical
flashlight and is very easy to use.
2) Aradigm
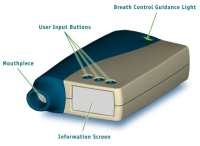 This system uses a hand-held inhalation device that is regulated with
microprocessors to produce a consistent dose using commercially available liquid
insulins. Liquid insulin is inserted into the device, and the aerosol delivers
particles 2–3 µm in size directly to the alveoli.4 The Aradigm system
circumvents any problems encountered in converting peptides into powders.
This system uses a hand-held inhalation device that is regulated with
microprocessors to produce a consistent dose using commercially available liquid
insulins. Liquid insulin is inserted into the device, and the aerosol delivers
particles 2–3 µm in size directly to the alveoli.4 The Aradigm system
circumvents any problems encountered in converting peptides into powders.
(Click on the image for a larger
view)
3)Tecnosphere insulin:
Recently, a new drug delivery system has been developed that
facilitates the absorption of peptides and proteins via the pulmonary route.
This system is known as Technosphere.6 The Technosphere insulin is an ordered
lattus array of Technosphere and recombinant human insulin.
Pharmacodynamic trials of this system have reported a rapid onset of metabolic
effect in a dose-dependent manner.6 Inhalation of Technosphere insulin was very
well tolerated.
In one study of Technosphere insulin involving 12 patients with type 2 diabetes,
the maximum metabolic effect was greater and the duration of action was shorter
with inhaled Technosphere insulin than was observed with subcutaneous regular
insulin. This small study concluded that inhaled Technosphere insulin may be
superior to regular human insulin administered subcutaneously for prandial
insulin supplementation in patients with type 2 diabetes because of its greater
onset, its shorter duration of action, its low within-subject variability, and
its convenience.7
Another small study of the Technosphere insulin system in healthy volunteers
reported high bioavailability (25.8% of that with subcutaneous and 14.6% of that
with intravenous administration) and an onset of action similar to that seen
with intravenous regular insulin. The study concluded that more research was
required to determine the feasibility of Technosphere insulin as a candidate for
future drug development.8
Long acting Pulmonary
insulin :
Several investigators have evaluated methods of prolonging insulin absorption
from the lungs of rodents.9 In one study, a porous aerosol particle containing
20% insulin and 80% poly(lactic acid-co-glycolic acid) was reported to
demonstrate sustained release of insulin into the blood over a period of several
days.10 Bioavailability of the inhaled particles was 87.5% of that with
subcutaneous injection of the sustained-release particles.
Unfortunately, the doses required in this trial were very
high. Doses of 9 mg of powder were administered to rats weighing 0.3 kg. This
would be roughly equivalent to a 2,100-mg dose for a 70-kg human—a mass
probably too large to be inhaled on a regular basis.
Inhalational
Systems:
1) AIR :
Recently, a unique, porous, dry-particle aerosol technology known as AIR was
developed with both fast-acting and slow-acting pulmonary insulin
formulations.11 AIR technology uses particles with a small aerodynamic size
(1–3 µm), a low density (<0.1 gm/ml), and large geometric particle size
(10–20 µm). These particles can be very easily aerosolized from a simple
inexpensive inhalation device, which effectively delivers the particles to the
deep lung and provides systemic absorption and high bioavailability.
The size and approximate shape of the AIR inhaler device is that of a standard
marker pen. The insulin is contained in blister packs. The inhaler requires no
power source and uses patients’ breath to deliver large amounts of powder with
a single breath.
The long-acting AIR insulin exhibits a pharmacokinetic profile similar to that
of human insulin (Humulin L), and the fast-release AIR insulin displays a
pharmacokinetic profile similar to that of human insulin in rats.11
Conclusion :
Several viable methods for the pulmonary delivery of
insulin are currently in development. Based on available data, pulmonary insulin
appears to be effective and safe.
While the majority of insulin delivery systems being
evaluated use rapid-acting insulin, systems are also being developed that may
allow for the administration of long-acting insulins. Ease of administration of
pulmonary insulin may lead to better compliance and better glycemic control in
the long run.
![]()