
New
Immunological test for the diagnosis of malaria :
Principle of
the test:
The ICT Malaria P.f. is an in-vitro immunodiagnostics test for the
detection of circulating Plasmodium falciparum histidine-rich protein-2
(Pf HRP-2) 1,2 in whole blood.
The test uses two antibodies specific for P.f. HRP-2 antigen. One of the
antibodies is attached to visible colloidal gold and impregnated into a sample
pad, while the second antibody is immobilised as a line across the test strip.
Approximately 10 ÁL of whole blood is added to the sample pad where lysis
occurs and PF HRP-2 antigen, if present, binds to the colloidal gold labelled
antibody. When Reagent A is added to the sample pad, blood and labelled antibody
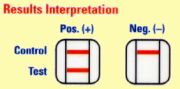
migrate up the test strip crossing the second antibody line. In a positive
sample, Pf HRP-2 complexed with the gold-labelled antibody is captured by the
antibody on the membrane and a pink line forms. In a negative sample, no pink
line forms.
Test
Procedure:
Results :
Additional
information:
1) The ICT Malaria P.f.
test will diagnose a positive infection 2 - 3 days after contracting the
disease.
2) The ICT Malaria P.f. test is
recognized and recommended by the World Health Organisation and has also been
approved by the Food and Drug Administration in the United States of America.
3) The ICT Malaria P.f. test
takes about 10 minutes to perform, from start to end result. Sterile lancets,
alcohol swabs and capillary tubes are provided with the tests and reagent in the
kit of 5.
4) For ultimate shelf life, the
ICT Malaria P.f. test should be stored at 2 - 8 degrees. The test will perform
at higher temperatures as long as they are not exposed to direct heat or
sunlight. The test should also not be performed in the near vicinity of a fan as
this cool dry out the test strip.
Current shelf life is in the region of 8 - 1 0
months. New generation test will have a shelf life of 24 months and storage of 4
- 30 degrees.
5) A New test that has just
been released is the ICT Malaria P.f. / P.v. test.
This test shall diagnose non -
specifically, i.e. it will give a single test line, for 3 further malaria's
being Plasmodium ovale, Plasmodium vivax and/or Plasmodium malariae. Should all
possible lines on the card be present in this test, then it is recommended and
suggested that the Plasmodium falciparum malaria be treated as first line, as
this is still the most dangerous form of malaria.
![]()